List Of Class 2 Narcotics
In the Federal Register of March 14, 2017 (82 FR 13609), FDA issued a notice proposing to exempt a list of class II devices from the premarket notification requirements under section 510(k) of the Federal Food, Drug, and Cosmetic Act (the FD&C Act) (21 U.S.C. 360(k)), subject to certain limitations. Most medications for type 2 diabetes are oral drugs. However, a few come as injections. Some people with type 2 diabetes may also need to take insulin. Alpha-glucosidase inhibitors.
Abstract
The Biopharmaceutics Classification system (BCS) classifies drug substances based on aqueous solubility and intestinal permeability. The objective of this study was to use the World Health Organization Model List of Essential Medicines to determine the distribution of BCS Class 1, 2, 3, and 4 drugs in Abbreviated New drug Applications (ANDA) submissions. To categorize solubility and intestinal permeability properties of generic drugs under development, we used a list of 61 drugs which were classified as BCS 1, 2, 3, and 4 drugs with certainty in the World Health Organization Model List of Essential Medicines. Applying this list to evaluation of 263 ANDA approvals of BCS drugs during the period of 2000 to 2011 indicated 110 approvals (41.8%) for Class 1 drugs (based on both biowaiver and in vivo bioequivalence studies), 55 (20.9%) approvals for Class 2 drugs, 98 (37.3%) approvals for Class 3 drugs, and no (0%) approvals for Class 4 drugs. The present data indicated a trend of more ANDA approvals of BCS Class 1 drugs than Class 3 or Class 2 drugs. Antiallergic drugs in Class 1, drugs for pain relief in Class 2 and antidiabetic drugs in Class 3 have received the largest number of approvals during this period.
INTRODUCTION
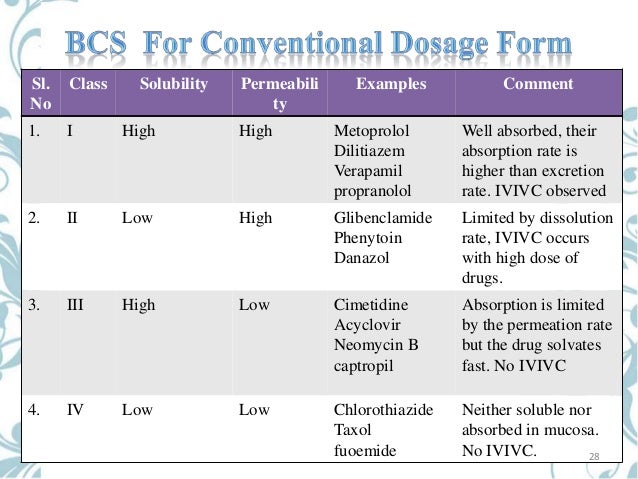
The Biopharmaceutics Classification system (BCS) classifies drug substances based on aqueous solubility and intestinal permeability which are the major characteristics of a drug substance that control its absorption in vivo (). According to the BCS classification approach, drug substances have been grouped into one of the four categories as Class 1 (high solubility, high permeability), Class 2 (low solubility, high permeability), Class 3 (high solubility, low permeability), and Class 4 (low solubility and low permeability). The US Food and Drug Administration (US FDA) implemented guidance based on BCS to waive in vivo bioavailability and bioequivalence study requirements to approve drug products (2). Biowaivers can be granted if the active pharmaceutical ingredient (API) is BCS Class 1, i.e., a drug substance of high solubility and high permeability, and if the immediate-release (IR) oral formulation exhibits rapid in vitro dissolution (2). The BCS-based biowaiver approval has also been adopted by the European Medicines Agency and World Health Organization (WHO) for IR oral drug products (). The WHO guidance recommends biowaivers for APIs that belong to BCS Class 1 and Class 3 and also certain APIs from BCS Class 2 (4). It is important to emphasize that the US FDA considers granting biowaivers only for Class 1 drugs but does not use the WHO BCS Class 1 list to grant such biowaivers. Rather, the US FDA follows the criteria described in its Guidance for Industry on the BCS (2) and considers the applicant's submitted solubility/permeability data on API and dissolution data on the drug product in deciding whether a Class 1 biowaiver is appropriate.
An in vivo bioequivalence (BE) study is the accepted test to ensure therapeutic equivalence of a generic product to its corresponding reference product. The BCS-based BE study waiver is becoming an important tool in approving generic drug products by US FDA. Initially, BCS-based BE study waivers were granted only for Scale-Up and Post Approval Changes (5). Later, this was implemented to approve generic IR oral drug products of BCS Class 1 API. BCS biowaivers are not granted to drug products with a narrow therapeutic range and drug products designed to be absorbed in the oral cavity. In vivo bioequivalence study waivers help to avoid unnecessary human exposure to drugs. In addition, biowaivers reduce the cost and time of developing generic IR oral drug products and also decrease regulatory burden ().
In the present study, to gain an understanding of the solubility and intestinal permeability properties of generic drugs under development in the USA, we examined the distribution of BCS Class 1, 2, 3, and 4 drugs in ANDA submissions based on the WHO Model List of Essential Medicines (EML) having sufficient solubility and permeability data for BCS classification with certainty (). The WHO Model List of Essential Medicines was used merely to describe generic drug solubility and intestinal permeability properties because it is publicly available and includes BCS Class 1, 2, 3, and 4 designations. However, as stated previously, to determine whether a generic drug is eligible for a BCS biowaiver, the FDA follows its own criteria set forth in its BCS Guidance for Industry (2).
METHODS
An internal FDA database was applied to identify generic drugs approved during the 2000 to 2011 period. For determining the distribution of BCS Class 1, 2, 3, and 4 drugs in ANDA submissions, we used the approved generic drug products of immediate-release oral dosage forms listed on the WHO EML having sufficient solubility and permeability data for BCS classification with certainty.
RESULTS AND DISCUSSION
We evaluated 263 approved generic drugs of IR products listed on the WHO EML to find out the distribution of BCS Class 1, 2, 3, and 4 drugs in approved ANDA applications during the 2000 to 2011 period. The WHO EML was used as it is a publicly available list of Class 1, 2, 3, and 4 drugs. Some of the FDA-approved oral IR ANDA products which are not listed in the WHO EML could not be considered for the study mainly due to insufficient solubility and permeability data for BCS classification with certainty. Of the 130 orally administered drugs on the WHO EML, 61 drugs have been classified as BCS 1 (21 drugs), 2 (10 drugs), 3 (24 drugs), and 4 (6 drugs) drugs with certainty (). ANDA approval data from 2000 to 2011 were analyzed to determine how many BCS Class 1, 2, 3, and 4 drugs from the list of 61 drugs were developed and approved as generic drugs based on both BCS biowaivers (applicable only for Class 1) and BE studies. The results indicated 110 (41.8%) approvals for Class 1 drug products, 55 (20.9%) approvals for Class 2 drug products, and 98 (37.3%) approvals for Class 3 drug products (Fig. 1). Figure 2 shows the yearly approval of different BCS Class generic drug products during this period. There were no approvals for WHO EML BCS Class 4 drug products during the 2000 to 2011 period. Thirty two different therapeutic classes of IR products of BCS Class 1, 2, and 3 were approved during this period. Of these 32 different therapeutics classes, antiallergic drugs in Class 1, drugs for pain relief in Class 2, and antidiabetic drugs in Class 3 have received the largest number of approvals.
The percent approval of different classes of BCS drugs listed on WHO EML from 2000 to 2011
ANDA approvals of WHO EML BCS Class drug products from 2000 to 2011 based on BCS biowaivers and in vivo BE studies
As stated above, the US FDA grants biowaivers based on the applicant's submitted solubility/permeability data on API and dissolution data on the drug product. We evaluated the quality of BCS biowaiver ANDA applications submitted to the FDA and noted some commonly occurring deficiencies. These deficiencies are mostly associated with solubility and permeability studies on API and are listed below:
****************** We’re always happy to hear from you! This step is required for mysms to work. This app works for phone users. Do you have a suggestion? *** Just follow these steps and you’ll be on your way: Step 1) Install mysms on your phone and register Step 2) Start the mysms Windows 8 app on your PC or tablet and log in using your number and password. Send texts from laptop.
Lack of multi-pH solubility profiles
Inappropriate method of solubility determination
Lack of dissolution data for all strengths
Missing standard operating procedures for analytical methods
Missing data supporting gastrointestinal stability
Lack of data on efflux transporter(s) in the cell line used for in vitro permeability
Lack of bidirectional in vitro permeability data on control model compounds
The above information is provided to assist applicants who submit BCS biowaiver requests to the FDA to prepare high-quality submissions. We hope that publication of this information on commonly occurring deficiencies associated with BCS biowaiver ANDA applications will promote application and review efficiency.
CONCLUSION
The data presented in this study on 32 different therapeutic classes of BCS generic drugs approved by the US FDA during the 2000–2011 period indicated the following ANDA approval trend: BCS Class 1 > Class 3 > Class 2, with no BCS Class 4 drugs evaluated. Antiallergic drugs in Class 1, drugs for pain relief in Class 2, and antidiabetic drugs in Class 3 have received the largest number of approvals.
List Of Class 2 Narcotics
References

List Of Schedule 1 Drugs
